It was really fun and cool.
What is Crystallization?
Basically, crystallization is the most common method used to purify soluble solids. There are many ways to carry out crystallization. The seeding method allows you to grow a large single crystal, others allow you to grow many crystals at a time. By controlling the variables such as cooling rate and evaporation rate, the size and shape of the crystals can be controlled.
Some conditions:
Firstly, the solid must be soluble in water. Secondly, the solubility of the substance should change with changing temperature. (eg solubility increases as temperature increases). Thirdly, the solution used should be saturated with the solute.
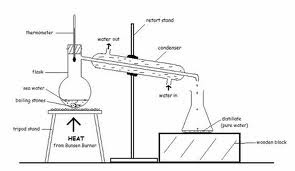
For the experiment we conducted, we purified copper (ll) sulphate crystals and investigate the effect of the cooling rate on the size of the crystals obtained.
Steps:
- Heat about 20cm3 of water in the beaker. Stop heating and remove from the tripod stand once bubbles are observed in the water or when the water boils.
- Add one spatula of copper (ll) sulphate into the hot water.
- Stir the mixture until the copper(ll) sulphate dissolves before adding another spatula of copper(ll) sulphate.
- Repeat Step 3 until no more copper(ll) sulphate can be dissolved
- Filter the solution if there are any solid impurities.
- Heat the copper(ll) sulphate in an evaporating dish.
- Stop heating when about half the solvent has evaporated from the solution. Do not heat the solution to dryness. (If a crust form,stop heating and add a little water)
- Pour the solution into a boiling tube and cool it in ice-cold water and measure the time taken for crystals to appear.
- Collect the crystals and dry them on filter paper
- Observe the crystals formed.
Step 6
Residue left after filtering the solution
Step 8
After a while of cooling (crystals are forming)
So there! The very awesome experiment.
Reflection: It was cool to see how crystallization was performed as this process is applied to daily life, to make sugar crystals that we eat. I think it is a tedious process though, to make sugar crystals.
End